
India has a huge livestock population supporting the socio-economics of the majority of the population. The country with a geographic area of 3,287,240 sq.km supports 300 million bovines, 65.07 million sheep, 135.2 million goats, and about 10.3 million pigs as per 19th livestock census. The cattle population is showing negative growth in recent censuses, mostly due to a decrease in the indigenous population. Despite the decrease in cattle population, the crossbred population of our country has shown increasing trends. There is an increase in coverage of artificial insemination (AI); still, about 60-70% conception is due to service by community bulls of the villages. India could achieve only 26 % of its ambitious target of 100 million AI(s) in 2017-18 due to various reasons like wastage of high-quality semen dosages, lesser efficiency of insemination workers and utilization ( 10 %) of available indigenous bull semen in the entire project (The Pioneer, 10th August 2017). Natural services by theses community bulls are responsible for many sexually transmitted diseases, that cause infertility in bovines. In a recent report on current status of Infectious Bovine Rhinotracheitis (IBR) in India by Chandranaik et al. (2016) emphasized the possible cause of spread of IBR from its first to in 1976 to 36% in his study was the adaption of crossbreeding policy to augment the milk production which resulted in the unhindered transmission of the virus. Undergoing the latency stage is characteristic of the virus causing IBR. Such latent virus carrier cattle remain silent shedders of the virus and a potent source for disseminating infection. The most perilous situation is unawareness and ignorance about this disease amongst livestock owners or the field veterinarians, AI workers, and para-vets. A survey conducted under a project by Indian Council of Agricultural Research-Research Center for Eastern region (ICAR RCER), Patna endorsed such statement as no farmer was aware of this disease in 8 villages of Bihar and four villages of Jharkhand (Fig.1). Thus the objective of this article is to disseminate awareness about this disease, its control and available treatment for mitigation amongst veterinarians, extension workers, and academicians.

Fig.1 Animal health awareness camp in Seikhpura district of Bihar
Infectious Bovine Rhinotracheitis (IBR) virus
IBR is caused by Bovine Herpes virus 1 (BHV1) belonging to the family Herpesviridae and the subfamily Alpha-herpes-virinae. It is a double-stranded DNA virus. The genome is reported to encode nearly 70 proteins, among which three glycoproteins, gB, gC, and gD can be of diagnostic importance. The BHV1 can be differentiated into two subtypes based on restriction endonuclease as BHV-1.1 (respiratory form) and BHV-1.2 (genital form). The respiratory form is more common and often referred to as infectious bovine rhinotracheitis or red-nose. BHV-1 shares specific biological properties with herpes simplex virus and capable of replication in the mucosal epithelium and latter establishing lifelong latency in ganglionic neurons of the peripheral nervous system. Long-term persistence and reactivation may occur within germinal centers of the pharyngeal tonsil. BHV-1 generally infects cattle higher than six months of age when the maternal immunity fails to persist. The virus is reasonably resistant in the environment. Virus infectivity is stable up to one month at 4°C. The virus is sensitive to many commonly used disinfectants like phenol derivate, quaternary ammonium bases, and formalin.
Epidemiology:
The first infection of BHV-1 was recorded in cattle in 1841 in Germany. However, it was isolated first in 1958. The seroprevalence of BHV-1 has been reported to vary from country to country and time to time from Africa, America, China, England, India, and several other countries. Countries like Denmark, Sweden, and Switzerland are officially free of IBR. In India, IBR was reported first from Uttar Pradesh in 1976. Currently, the disease is prevalent in most states of India covering South to North and East to West (i.e., Bihar, Kerala, Karnataka, Tamil Nadu, Gujarat, Odissa and Nagaland). Both morbidity and mortality are generally low. An exhaustive study carried out in different dairies of India reported that the IBR antibodies were widely prevalent in all regions of the country ranging from 36.5% -84.5% with an overall prevalence of 61.6%. ICAR RCER, Patna has reported seroprevalence of 6.1% in serum samples of bovine of Bihar.
Susceptible Host: Cattle is the primary host of BHV-1, however other animals like goats, sheep, buffaloes, camel, elephant, wild species may be infected and harbor the virus and play a role in maintaining the virus.
Source of Infection and Transmission:
All ages of animals are potentially at risk. The virus is mainly transmitted from an infected animal to uninfected susceptible cattle by direct contact with respiratory, ocular, or genital secretions. IBR virus quickly spread through a herd of calves. The secretions of affected calves are highly infectious and may last for 10-14 days.
Another important source of infection is the infected bull and its semen. The virus gets mechanically transmitted between bulls during semen collection. Infected semen can also act as a source of infection to a susceptible female by artificial insemination and often is the main route of infection of the virus, causing Infectious Pustular vulvovaginitis. Infected semen may also be responsible for reduced fertility and abnormal fetal development in cyclic animals.
Most infected cow and bull become latent, and the virus persists in trigeminal or sacral ganglia. It may again start to shed the virus when exposed to adverse environments or compromised health.
Disease Manifestation
IBR virus causes respiratory and reproductive symptoms and often multi-systemic diseases associated with secondary complications. The incubation period may vary up to 21 days. IBR infection can be clinical, subclinical, and latent. The clinical symptom of IBR is fever with a severe to mucopurulent nasal discharge, necrotic lesions in the nose, conjunctivitis due to respiratory tract afflictions. A nasal discharge, along with nasal congestion, may develop and is referred to as “red nose.” Fetal abortions may also occur after respiratory infections. BHV1 may follow infection with other secondary bacterial/viral infections (Bovine parainfluenzavirus, bovine respiratory syncytial virus, Mannheimia haemolytica, Pasteurella multocida, and Histoplasma somnis) resulting in bronchopneumonia or bovine respiratory disease complex or Shipping Fever. The eyelids may get swollen with conjunctivitis, and there may be ulcers on the nose in severe cases. There is halitosis from pus in the larynx and trachea, and varying degrees of dyspnoea. Inappetence, weight loss, and milk drop often occur and can be severe. BHV1 also causes infectious pustular vulvovaginitis (IPV) and balanoposthitis (IBP). Bulls may shed the virus in their semen during both clinical and subclinical infections. IPV in the cow or IBP in bulls causes pustular lesions of the genital tract and may lead to abortion in a cow. IPV is observed 1-3 days after mating. The first sign of IPV are frequent urination and followed later by small pustules (1-2 mm) on the vulva (Fig. 2).
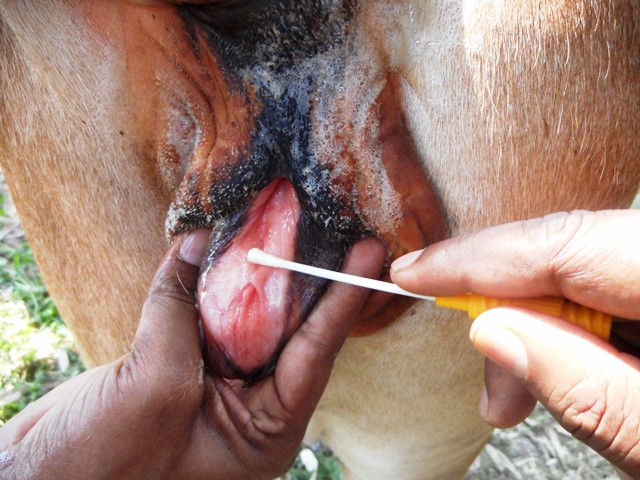
Fig.2 Papules in the mucosa of vagina suspected of IPV with a recent history of natural mating with village bull and repeat breeder.
IPV infection usually does not penetrate to the cervix or uterus, and thus, abortion is rare. If artificially inseminated using infected semen is done directly into the uterus, it may induce endometritis, disrupt the cow’s estrous cycles, and reduces its fertility.
Bulls with IBP have reduced libido, painful erection and ejaculation with the presence of pustules or ulcers on the mucosal surface of the penis and prepuce.
Conjunctivitis is also manifested as a result of the involvement of other pathogens resembling “pink eye” caused by Moraxella bovis but can be differentiated based on severe conjunctivitis and edema of the cornea near the corneoscleral junction and lack of corneal ulcer (Fig. 3). Sometimes it is the sole clinical manifestation.
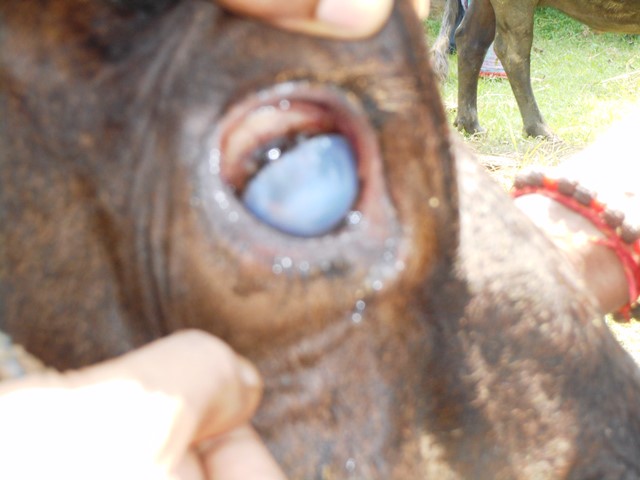
Fig3. Conjunctivitis without an ulcer in a suspected case of IBR in Sheikhpura district of Bihar
Diagnosis
The main objective of diagnosis is to know the disease incidence, for adopting control measures and for certification of bulls free of this disease. Disease diagnosis in affected cattle may not be yielding based on clinical symptoms and history. However, the history of use of local bull for natural service, symptoms described above may be suggestive for further investigation. Assessment of BHV-1 infection in a population is by indirect detection of the host response to the pathogen based on serum antibody and most specific by direct detection of the virus.
The serological tests commonly used for detecting antibodies of the virus are virus neutralization (VN) test, fluorescent antibody technique, and Enzyme-linked Immunosorbent Assay for Antibody (ELISA). Serum antibodies to the virus are detected within 1-21 days of infection. Maternally-derived antibodies usually disappear in about 4–5 months.
BHV1 being a virus can be confirmed by its isolation in a laboratory by inoculating in susceptible cell cultures like Madin Darby Bovine Kidney (MDBK) cell lines, bovine turbinate line, etc. Usually, virus isolation is possible from samples ( collected in suitable media ) such as swabbing from lesions in nasal or reproductive tracts.
Recently many molecular biology techniques like Polymerase chain reaction (PCR), real-time PCR (qPCR), Southern blot hybridization and labeled probes, etc. are considered very sensitive, rapid, independent of sample quality and useful in the diagnosis of BHV1. Various diagnostic genes for exploring this virus are glycoprotein-encoding genes (gB, gC, and gE), BHV1 thymidine kinase (tk)-coding gene and UL36 genes, etc.
Treatment
There is no direct treatment for viral diseases, including IBR. BHV, as all other viruses are, is incapable of replicating without a host cell. This unique feature of viruses makes it difficult to design a treatment to kill the virus pathogen selectively.
However, an infected IBR cow or bull should be isolated from the rest of the herd and treated with anti-inflammatory drugs and if necessary broad-spectrum antibiotics may be given parenterally for preventing secondary infection. Additional treatment includes the use of multi-vitamins and provision of adequate ration and comfortable stress-free environment. Effective anti-herpes drugs, such as Acyclovir, Ganciclovir, and Vidarabine, are available for treatment in human medicine but not in veterinary practice. IBR tends latency; no prophylactic antiviral treatment is capable of reducing the disease incidence and recovery from latent infection.
Research to alleviate this virus in cattle focuses on the development of antiviral recombinant immunotoxin and herbal medicines. Herbal and purified products provide a precious resource for novel antiviral drug development; however, most studies are limited to Herpes simplex virus 1 in cell cultures. Many compounds (tannins, flavones, alkaloids) extracted from various species of plants have shown antiviral in vitro activity against a spectrum of viruses. Anthraquinones from extracts of Aloe vera, Rhamnus frangula, Rheum officinale, and Cassia angustifolia has been reported to be useful against HSV-1. Cassia javanica, Melia azedarach, Phyllanthus urinaria, roots of Achyranthes aspera, Basella rubra and Swertia chirata plant extract reported having antiviral properties against DNA virus including Herpes virus.
Control Measures:
Prophylactic vaccination is the most suitable means to reduce the incidence of disease. Timely diagnosis, isolation, and removal of positive animals, and trade-related biosecurity are few essential elements of control measures. Various kinds of vaccines available in the global market to be used in cattle against BHV1 infections are modified live virus vaccines, inactivated vaccines, subunit vaccines, and marker vaccines. It can be given at the age of 4 months and above and needs to be repeated at six monthly intervals. In the case of a clinical outbreak to all susceptible bovine populations should be vaccinated within the area.
Vaccine development in India is underway with many research groups in different organizations. However, a commercial vaccine for use by farmers has not been launched or marketed. Nonavailability of vaccine and also low-cost diagnostic kits is the key constraint in control of IBR. First reports on IBR vaccine development by BIAF and technology transferred by the National Research Development Corporation, New Delhi to the Pune-based Hoechst Roussel Veterinary Pvt Ltd for its commercial production published on 5.07. 2001 by Business Line. However, it is still not available for use in the market. Policy paper 46 on Veterinary vaccine and diagnostic (2010) published by the National Academy of Agricultural Sciences, New Delhi has suggested marker vaccine with gE deletion mutant strains of BHV1 as a vaccine of choice. Till recently the vaccine for IBR was mostly imported from Europe, USA, etc., eg. Bovilis® IBR Marker Live. The cost of the vaccine with the imported source is approx US$ 1.0 per dose.
Carrier cattle are to be isolated from the herd. Hygienic measures and isolation or quarantine for 21 days should be mandatory before the introduction of a new cow or bull in the herd. A country must implement strict import restrictions on cattle, semen, and embryos from BHV1 infected countries.
You can help us to improve the content of this page. Please post your comments or share with us any resource linked to IBR and India.
Further Reading:
- Saurabh Majumder, MA Ramakrishnan, S Nandi “Infectious Bovine Rhinotracheitis: An Indian Perspective” Int.J.Curr.Microbiol.App.Sci (2015) 4(10): 844-858 Click Here
- IBR page of Dairy Knowledge Portal of NDDB Click Here
- A Chatterjee, S Bakshi, S N Sarkar, J Mitra, and S Chowdhury “Bovine Herpes virus-1 and its infection in India – a Review” Indian J. Anim. Hlth. (2016), 55(1) : 21-40 ( IBR_IndiaReview_2016_Chatterjee_etal )
- Chandranaik, B. M., Patil, S. S., Rathnamma, D., Suresh, K. P., & Reddy, G. B. (2016). Infectious Bovine Rhinotracheitis (IBR): Current Status in India. Indian Journal of Comparative Microbiology, Immunology, and Infectious Diseases, 37(1), 1-4. Click Here
- Kaur G, Dwivedi PN, Verma R and Sharma NS (2013) Prevalence of Bovine Herpesvirus-1 (BHV-1) in cattle and buffaloes in Punjab, Vet. World 6(6):343-345, doi:10.5455/vetworld.2013.343-345 Click Here
- Ludwig, H., & Gregersen, J. P. (1986). Infectious bovine rhinotracheitis-infections pustular vulvovaginitis: BHV-1 infections. Revue Scientifique et Technique de l’OIE (France). Click Here
- Queiroz-Castro, V. L. D., da Costa, E. P., Alves, S. V. P., Guimarães, J. D., Dohanik, V. T., Santos, M. R., … & Silva-Júnior, A. (2019). Detection of bovine herpesvirus 1 in genital organs of naturally infected cows. Theriogenology. 130, p.125. Click Here
- Caldow, G., Geraghty, T., Mason, C., Carty, H., & Wilson, D. (2018). Bovine herpesvirus 1 infection in cattle: a discussion on vaccination and control. Livestock, 23(3), 110-115. Click Here
- Romero-Salas, D., Cruz-Romero, A., Aguilar-Domínguez, M., Ibarra-Priego, N., Barradas-Piña, F. T., Domingues, N. L., ….. & de Leon, P. A. (2018). Seroepidemiology of Bovine Herpes Virus-1 Infection in Water Buffaloes from the state of Veracruz, Mexico. Tropical Biomedicine, 35(2), 541-552. Click Here
- Hidayati, D. N., Untari, T., Wibowo, M. H., Akiyama, K., & Asmara, W. (2018). Cloning and sequencing gB, gD, and gM genes to perform the genetic variability of bovine herpesvirus-1 from Indonesia. Veterinary World, 11(9), 1255. Click Here
- McManus, C., Guimarães, R., Gianezini, M., & Zago, D. (2016). Dynamics of Cattle Production in Brazil. PLoS One, 11(1), e0147138. Click Here
- Deregt, D., (1998). Infectious bovine rhinotracheitis and bovine viral diarrhea: Repercussions for animal health and international trade. https://www.oie.int/doc/ged/D5645.PDF Click Here
- Common animal diseases and their management – Vikaspedia. Click Here
- Queiroz-Castro, V.L.D., da Costa, E.P., Alves, S.V.P., Guimarães, J.D., Dohanik, V.T., Santos, M.R., de Souza, L.F.L., Ribeiro, C.G., Caldas, R.T. and Silva-Júnior, A., 2019. Detection of bovine herpesvirus 1 in genital organs of naturally infected cows. Theriogenology. Click Here







